APES Group
| Introduction | Jiri Klimes | Research | Members | Theses available | Teaching | Publications |
We are interested in understanding processes at nanoscale and in the development of methods to describe such processes. This is a field between chemical physics, surface science, materials science, and related research areas. The methods we employ and develop are density functional theory, many-body perturbation theory as well as traditional quantum chemistry, which we try to apply to large, usually periodic, systems.
You can read more about our ERC-funded project APES here.
Here are some examples of our work.
|
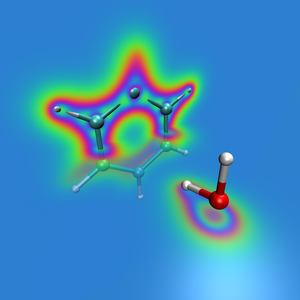
Weak interactionsWeak intermolecular or non-covalent interactions are important in many industrial and natural processes. However, their accurate description presents a challenge for many quantum chemical methods. For example, they are not described by the Hartree-Fock method nor by standard semi-local or hybrid exchange-correlation functionals of the density functional theory. Several ways have been developed to describe weak interactions but there are still many open questions concerning, e.g., the reliability of these methods to different systems. |
|
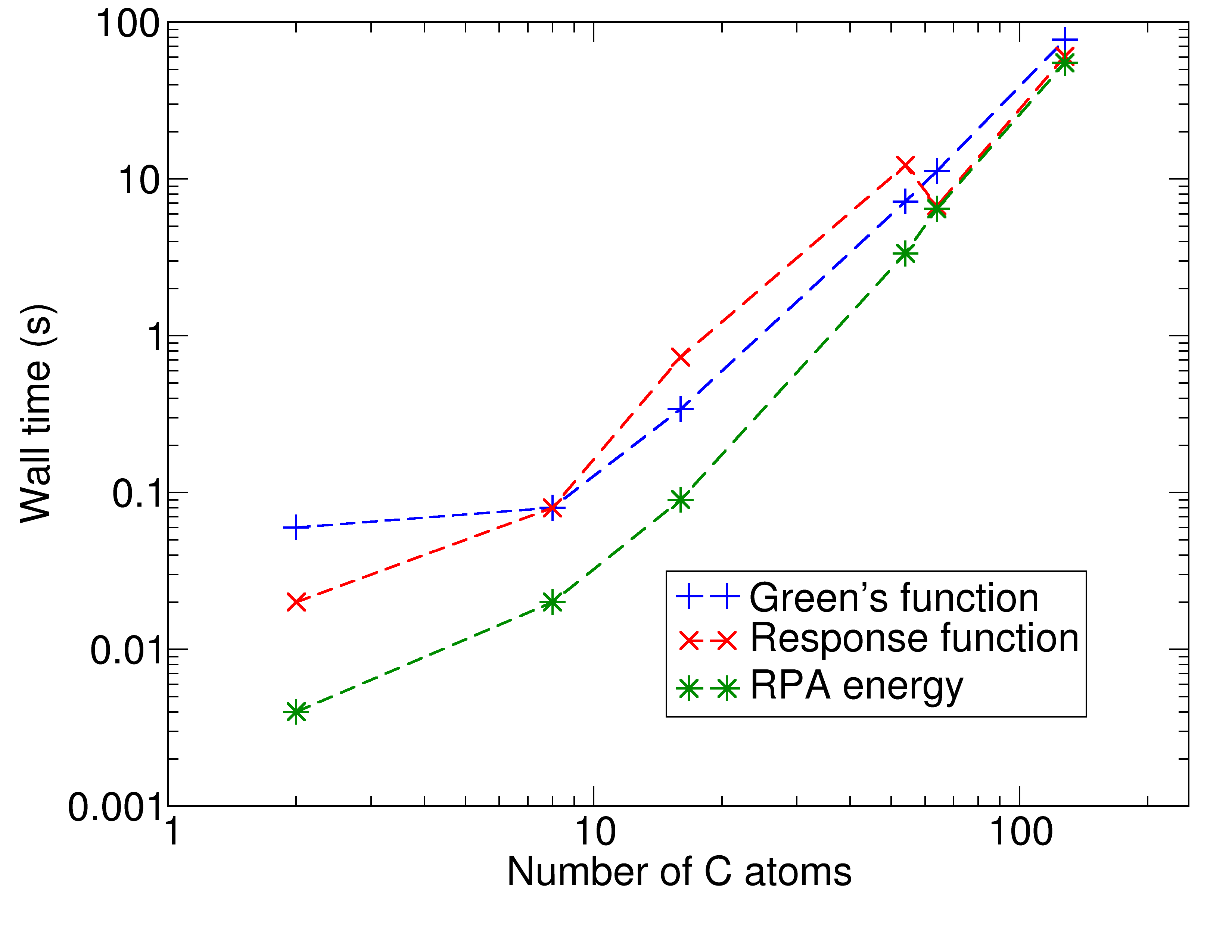
Implementation of improved algorithmsOne of the big issues that occur when we apply accurate methods is their unfavourable scaling with the system size. For very accurate methods, increasing the system by a factor of two can lead to an increase of computational time by a factor of 10 to 100 or even more. Devising and implementing clever algorithms is one way of reducing this prohibitive scaling. In the last few years, I've contributed to the development of a code that reduces the scaling of one promising method, the RPA, from O(N4) to O(N3). This allows us to study systems with hundreds of atoms on a routine basis. |
|
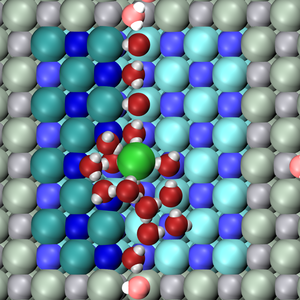
Natural and industrial processesDissolution of table salt (NaCl) is a common everyday process which is relevant to many other natural processes where water interacts with materials. In this project which I did during my PhD, I looked at the initial steps of salt dissolution, when the surface is not covered by a complete water layer. We found that undercoordinated ions on steps are more easily removed from the crystal. Rather surprisingly, Cl ion, is removed first, although its interaction with water molecules is weaker than the interaction of Na ion with water. Removing Cl ion creates a site with several exposed Na ions which is a very favourable adsorption site for water molecules. |